
- Company
- Public Sector
- Insights
Search
Integrated, AI based Safety Analytics & Compliance Automation Platform
Enabling Safety of Drugs, Vaccines, Devices and Cosmetics

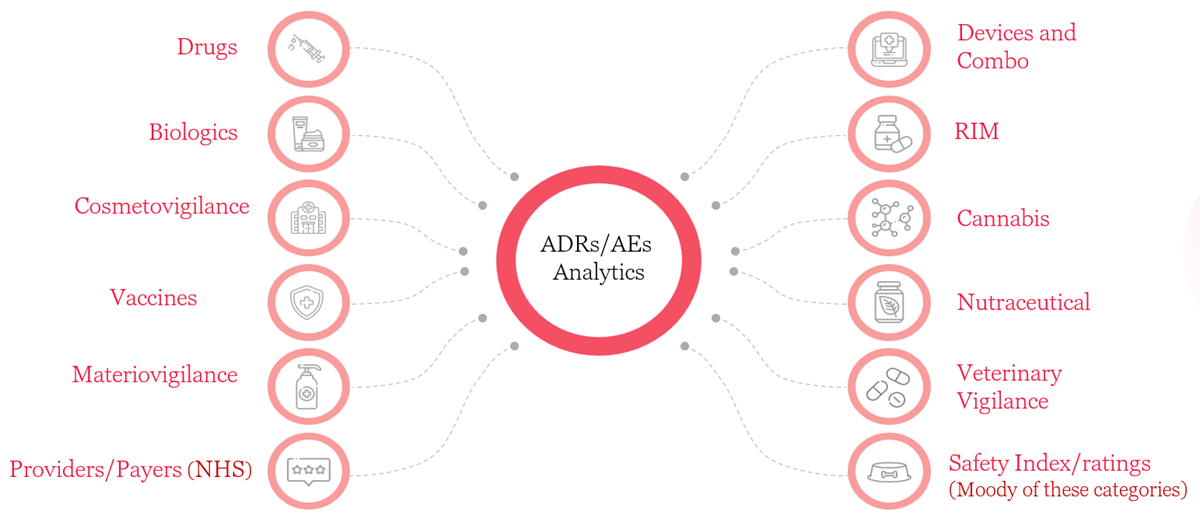
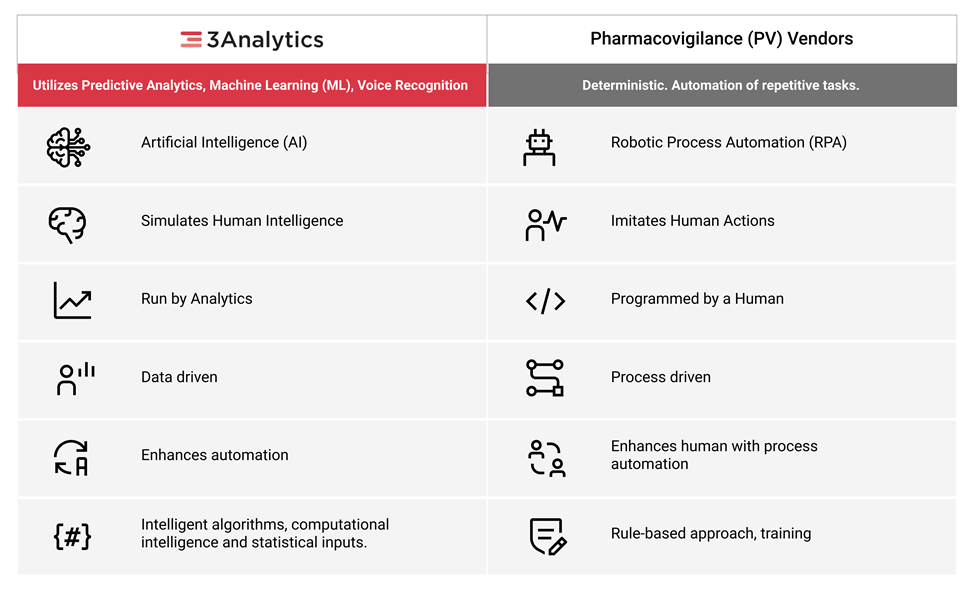
Bringing a specialized AI/ML based integrated platform powered by "3Analytics" to automate end-to-end safety compliance with data aggregation, workflow automation and predictive analytics.
The components are the following:
- Multilingual device complaints process automation from
multiple sources - IMDRF coding automation
- Algorithm driven signal detection
- Literature surveillance for Clinical evaluation reports
IMDRF coding automation
Global medical device leaders have been adopting 3Analytics for IMDRF coding for upstream needs followed by downstream signal detection to comply with the EU MDR regulations. The algorithm codes both device complaints and MedDRA codes for associated adverse events in addition to Annexure E of IMDRF coding. This is now being integrated into multiple QMS systems in the industry namely Catsweb (AssurX) and Trackwise (Sparta Systems-Honeywell).
The AI Platform automates this data conversion process and integrates with an algorithm driven Signal Detection and Management application, meeting new guidelines- reliably and cost effectively.
This integrated solution offers an easier pathway, reducing the noncompliance risks. It becomes even more critical for big manufacturers who may have a large legacy data set and hence manual conversion may not be an option.

- Reduce vaccine hesitancy using facts & data
- Data-driven early warning system
- Built by Experts in Artificial intelligence & Public health
- Predicts Adverse Events and High-Risk Patients
- Designed in consultation with JHPIEGO

- Company
- Public Sector
- Insights
© 2023 I&I Software. All Rights Reserved.

